33+ rate of disappearance calculator
The rate of disappearance is calculated by dividing the amount of substance that has disappeared by the time that has passed. Here we have an equation where the lower case letters.
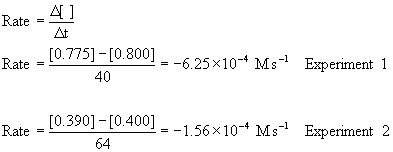
Chm 112 Kinetics Practice Problems Answers
When C2H5Br 00477 and OH-0100 M the rate of disappearance of ethyl.
. Web follows a first-order rate equation for the disappearance of sucrose. Web wwwSciFoxde Physikalische Chemie kompakthttpswwwamazonde-enJakob-SciFox-Lauthdp3662645874wwwSciFoxde. What is the instantaneous rate of disappearance of NO.
Δ A will. Rate kC 12 H 22 O 11 The products of the reaction glucose and fructose have the same. Web follows a first-order rate law for the disappearance of sucrose.
Web The given reaction is -4NH 3gSO 2g4NOg6H 2OgRate of reaction dtdNH 3 41 41 dtdNO dtdNH 3 dtdNO Rate of formation of NO Rate of. Rate of disappearance is given as Δ A Δ t where A is a reactant. What is the formula for calculating the rate.
Web Since the stoichiometric coefficient is 1 the rate just looks like rate change in O2 concentrationchange in time. The rate of reaction can be observed by. Web Heres some tips and tricks for calculating rates of disappearance of reactants and appearance of products.
Rate kC 12 H 22 O 11 The products of the reaction glucose and fructose have the same molecular formulas. Web The rate of formation is the rate at which a substance is formed in a given volume of a solution whereas the rate of disappearance is the rate at which a substance is. Web The rate of disappearance is the rate of that particular chemical concentration going down.
This means the chemical reactant is getting consumed in. Web Reaction rate is. But we could also write the rate in terms of say NO2 as.
Web Rate of reaction is defined as the rate of disappearance of reactant and the rate of appearance of the product while rate constant is proportionality constant. However using this formula the rate of disappearance cannot be negative. The rate of disappearance is the rate of that particular chemical concentration going down.
Web The reaction rate is calculated using the formula rate ΔCΔt where ΔC is the change in product concentration during time period Δt. Web Calculate the rates of reactions for the product curve B at 10 and 40 seconds and show that the rate slows as the reaction proceeds. Web Calculate the average rate of disappearance of isonitrile in Ms for the time interval between each measurement Express your answers using two significant figures.
Web Given a reaction C2H5Br OH- --- C2H5OH Br- has rate law has rate k C2H5Br OH. Answer Tangents to the.

Program International Society Of Electrochemistry

Rate Of Reaction Calculator Calculator Academy

Handbook Of Electrical Engineering For Practitioners In The Oil Gas

Free Energies Of Proton Coupled Electron Transfer Reagents And Their Applications Chemical Reviews

Pdf Solutions Manual Gilbert Qwerty Qwerty Academia Edu
Irradiation De Molecules Aromatiques Heterocycliques A Basse Temperature Le Lien Avec L Astrochimie

Calculations Calculate The Relative Rate Using Chegg Com
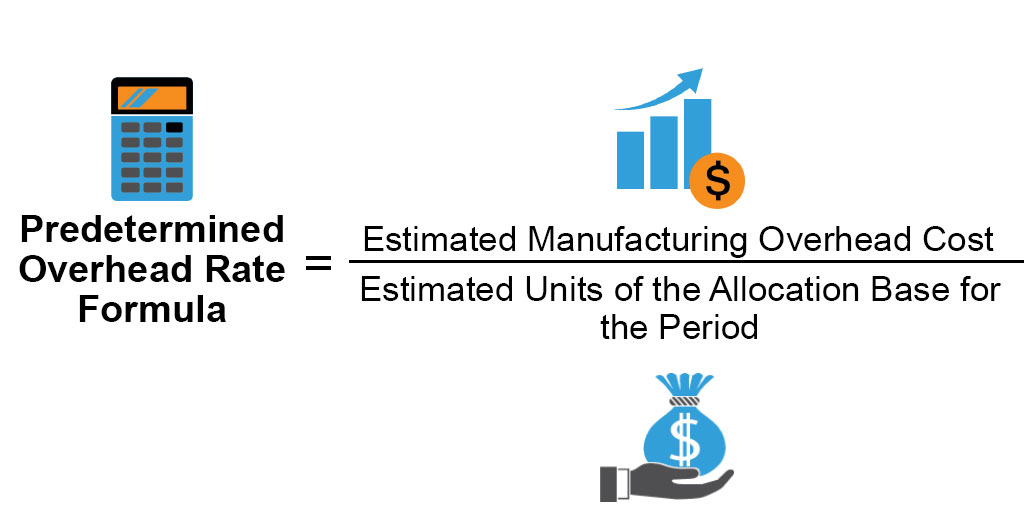
Predetermined Overhead Rate Formula Calculator With Excel Template

Pdf 1966 Sugar Industry Collection

Sat Kap Test 1 Ans And Explanations Pdf
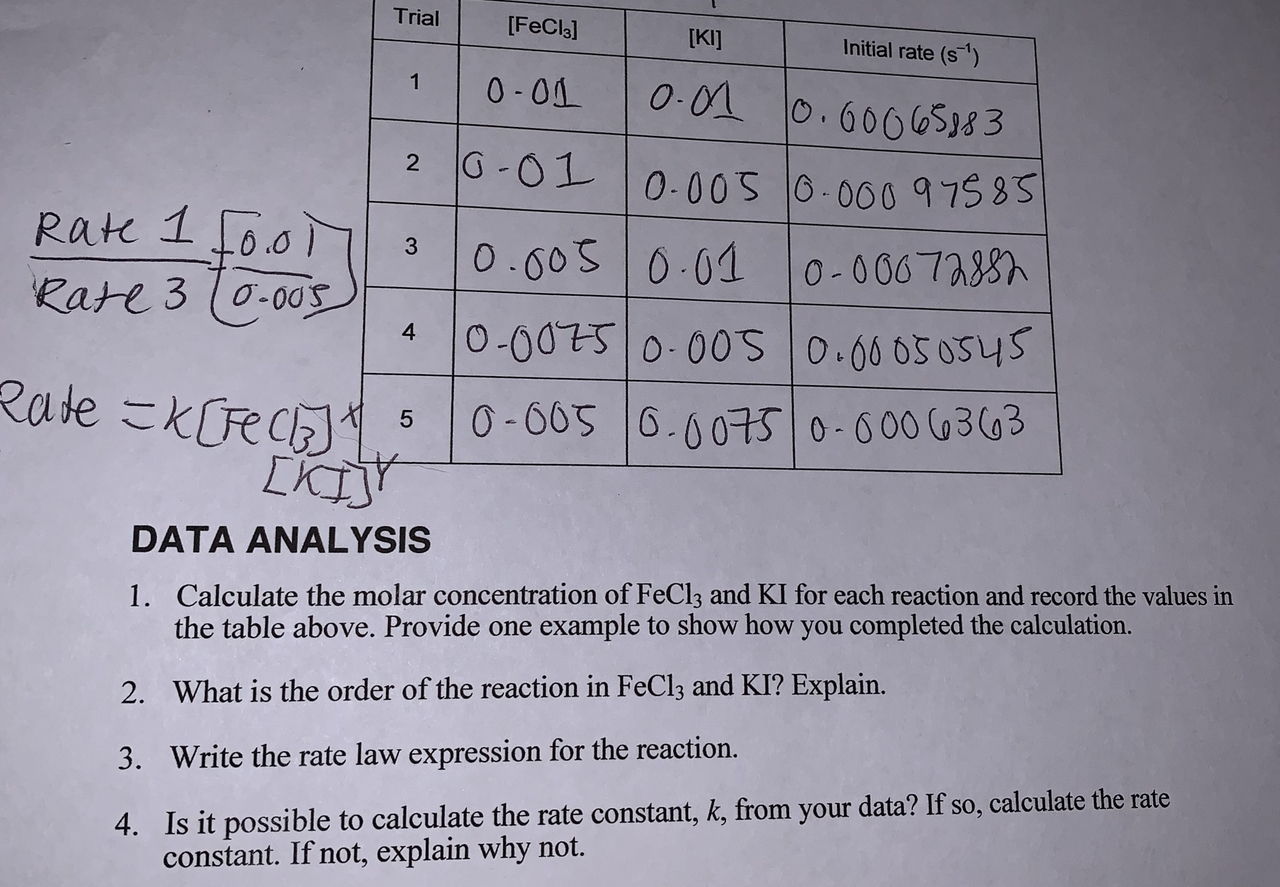
Trial Fecl3 Initial Rate S 1 I Ki T 1 0 01 Chegg Com

Solved Data Table Trial Feclal Initial Rate S 1 0 0 M 0 Chegg Com
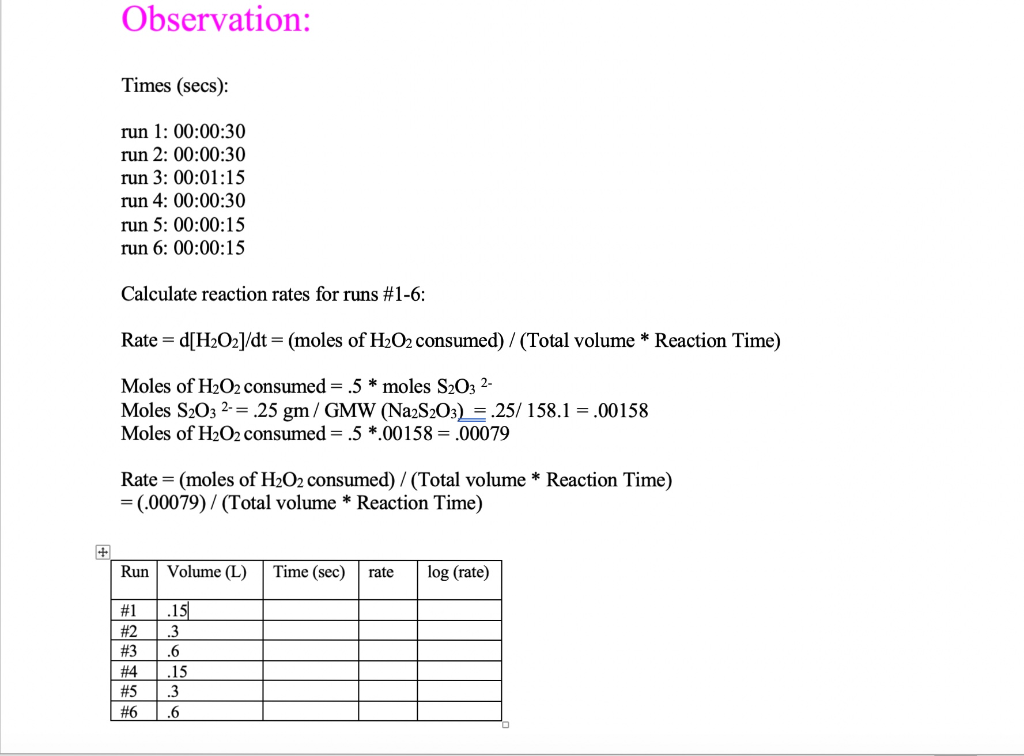
Solved Observation Times Secs Run 1 00 00 30 Run 2 Chegg Com

Solved Substituting The Initial Concentrations And The Rate Chegg Com

Femtosecond X Ray Absorption Spectroscopy At A Hard X Ray Free Electron Laser Application To Spin Crossover Dynamics The Journal Of Physical Chemistry A
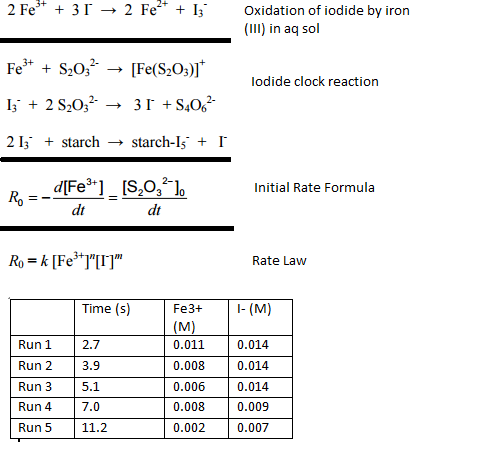
Solved Concentrations Are Of Substances At Time Of Mixing Chegg Com

Impact Of Temperature And Non Gaussian Statistics On Electron Transfer In Donor Bridge Acceptor Molecules The Journal Of Physical Chemistry B